New Implantable, Stretchable Bioelectronics Deliver High Performance Despite Strain
Interconnected & Integrated Bioelectronics Lab/UCLA
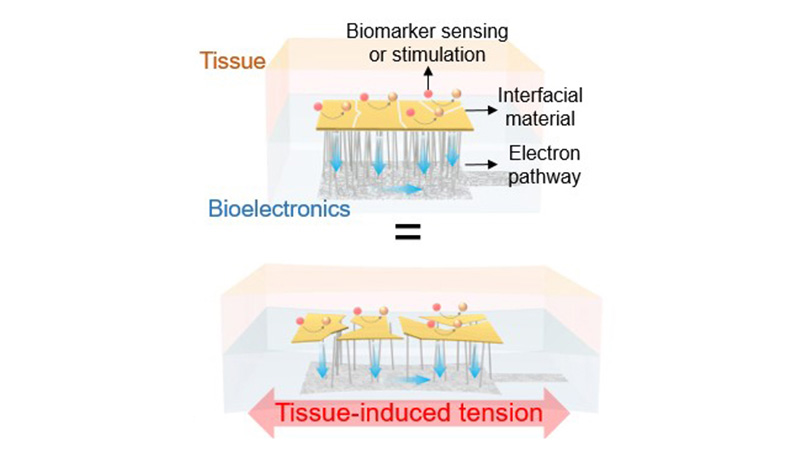
Schematic of a high-performance and strain-resistant bioelectronics material developed by UCLA researchers
A multidisciplinary team of engineers and doctors from UCLA has developed a new class of bioelectronics that can flex and bend inside biological tissues without compromising their sensing and stimulation performance.
The advance could apply to durable and ultrathin devices such as medical-grade bioelectrodes. The superior softness and stretchability of the electronics could significantly limit tissue damage and pave the way for their application in a broader range of medical procedures, including implants in sensitive areas such as the brain. Science recently published a study outlining how the devices are built and their performance on animal models.
“Electronics placed inside soft and squishy tissue should have the same pliability. But the current best options with matching mechanical properties suffer from strain-related distortions to the electrochemical and electrical performance, or the electrodes can become corroded when directly interfacing with tissue,” said study corresponding author Sam Emaminejad, an associate professor of electrical and computer engineering, and of bioengineering, at the UCLA Samueli School of Engineering. “Our solution overcomes these limitations and also provides a new way to construct long-lasting bioelectrodes with clinically proven high-performance materials, including those that are brittle.”
Key to the new technology is a three-layer framework. The outer layer that touches tissue is a thin film of clinically established, superior interfacial materials such as gold, platinum, carbon or iridium-oxide. The innermost layer is composed of electricity-conducting silver nanowires packed within a rubber-like polymer. A conductive adhesive film forms the middle layer and connects the outer and inner structures.
“Electronics placed inside soft and squishy tissue should have the same pliability. But the current best options with matching mechanical properties suffer from strain-related distortions to the electrochemical and electrical performance, or the electrodes can become corroded when directly interfacing with tissue,” said Sam Emaminejad.
This composite accommodates the eventual cracking of the metal surface film into smaller pieces as the electrodes naturally flex or stretch inside tissue. The broken pieces manage to maintain their electrical connection to the underlying silver nanowires through the intermediary strain-isolated vertical conduction path. The electrical resistance of the silver nanowires is also minimally impacted, realigning as a network and preserving electron flow. This ensures that the electrical operating points and the subsequent electrochemical reactions at the interface with tissue remain the same.
“In our approach, we achieve strain insensitivity by actually embracing the cracking of the materials — a phenomenon that is often avoided in the conventional designs of the stretchable devices,” said lead author and recent doctoral graduate Yichao Zhao, who was a member of Emaminejad’s Interconnected & Integrated Bioelectronics Lab at UCLA. “Our design strategy ensures electronic signals that flow between bioelectronics and tissue are insensitive to the strain caused by tissue — meaning the device’s sensing and stimulation capabilities can remain consistent throughout a medical procedure, or for the lifetime of an implant.”
The researchers built an array of stretchable, highly conductive and strain-insensitive bioelectrodes with different interfacial materials. They used the bioelectrodes to make sensors for detecting biomarker molecules and modulating the peripheral nerves of living mice.
The other co-lead authors are former UCLA postdoctoral researcher Bo Wang and current doctoral student researcher Jiawei Tan in Emaminejad’s lab. Senior authors on the paper comprises Dr. Benjamin Wu, a professor emeritus of bioengineering and dentistry; Hilary Coller, a professor of molecular cell and developmental biology, and of biological chemistry; Dr. Daniel Lu, a professor of neurosurgery; and Qibing Pei, a professor of materials science and engineering.
The research was supported by the National Institutes of Health, the National Science Foundation and an award from the UCLA Broad Stem Cell Research Center. The authors are applying for patents related to the work through the UCLA Technology Development Group.
