Handheld Diagnostic Lab Offers Point-of-Care Solution for Future Pandemics
Cost-saving advance can fully automate pooled testing and detect multiple diseases
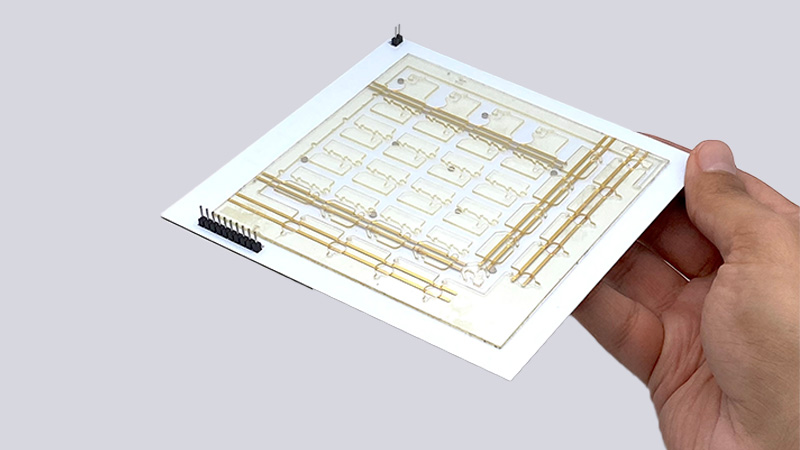
Courtesy of Kiarash Sabet/UCLA
UCLA-developed handheld diagnostic lab kit capable of fully automated multiplexed and pooled testing
Using swarms of pinhead-sized magnets inside a handheld, all-in-one lab kit, UCLA researchers have developed a technology that could significantly increase the speed and volume of disease testing, while reducing the costs and usage of scarce supplies.
The automated tests can be easily manufactured, deployed and performed timely at a doctor’s office, health clinic or at mass testing sites in airports and schools at the onset of any major infectious disease. The UCLA-led research team was motivated by the lack of equitable access to testing during the early months of the COVID-19 pandemic when only a handful of clinical laboratories were authorized to run tests. The technology breakthrough could help the authorities better prepare for future pandemics by decentralizing testing and maximizing the use of resources.
UCLA Samueli School of Engineering professor Dino Di Carlo of bioengineering and associate professor Sam Emaminejad of electrical and computer engineering co-authored a study, which was published this week in Nature. The paper outlined how the lab kit works and included findings from a clinical study with test samples from individuals who experienced COVID-19 symptoms. More than 100 test results using the lab kit were compared to the same samples tested for COVID-19 using polymerase chain reaction (PCR)-based molecular diagnostics performed as part of UCLA Health’s routine clinical care.
“Our handheld lab technology could help overcome some of the barriers of scarcity and access to tests, especially early in a pandemic, when it is most crucial to control disease spread,” said Emaminejad, who also holds a faculty appointment in bioengineering. “And beyond its potential to address issues of short supplies and high demand, it could be broadly adapted to test for many types of diseases in field and with lab-grade quality.”
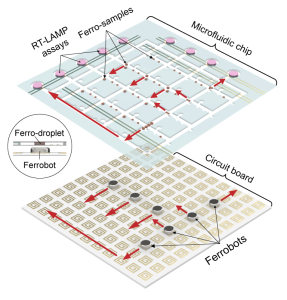
Using a circuit board that controls a set of movable, 1-millimeter-sized magnetic discs known as “ferrobots” to transport samples through the diagnostic workflow of a nucleic acid amplification test (NAAT), the researchers’ ultra-sensitive lab kit was able to detect the presence of genetic material from a virus — in this case, SARS-CoV-2 that causes COVID-19. The steps to separate, sort, mix and amplify testing samples are all automated and performed at a miniaturized level inside the kit.
“This platform’s compact design and automated handling of samples enable easy implementations of pooled testing where you can test dozens of patient samples at the same time, and all with the same materials it currently takes to test just one patient,” said Di Carlo, who holds UCLA’s Armond and Elena Hairapetian Chair in Engineering and Medicine. “For example, you could test students in an entire college residence hall with just a few dozen test kits.”
By designing the kit for pooled testing, the system requires much lower amounts of reagent chemicals than those needed for testing the samples individually. Up to 16 samples were combined and tested at once in the team’s study. If the pooled test showed a positive result, subsequent tests would automatically take place within the same platform until the actual positive samples were identified. This entire process took between 30 to 60 minutes, depending on whether there were positive samples. Thanks to the technology’s assay miniaturization and pooled-testing capabilities, the chemical reagent costs could be reduced by 10 to 300 times.

Aside from being able to test for several diseases simultaneously, the platform also offers precision and robust automation. For example, in a pooled-testing with 16 samples, more than 300 lab operations, including mixing and sorting, were automated by the ferrobots — that is more than 3,000 individual movements, or actuations. In their reliability studies, the researchers showed that the ferrobots could perform more than 8 million actuations without mistakes.
In addition to Di Carlo and Emaminejad, who are both faculty members of the California NanoSystems Institute at UCLA, the lead authors on this study are UCLA postdoctoral researcher Haisong Lin, and graduate students Wenzhuo Yu and Kiarash Sabet — all are members of Emaminejad’s Interconnected & Integrated Bioelectronics Lab at UCLA.
Other authors are UCLA engineering graduate students Michael Bogumil, Jacob Hambalek and Shuyu Lin; and postdoctoral researcher Yichao Zhao; as well as UCLA Health’s assistant clinical professor Sukantha Chandrasekaran and associate clinical professor Omai Garner of pathology and laboratory medicine.
The research was supported by the W.M. Keck Foundation’s COVID-19 Research Award Program, the National Science Foundation and the Precise Advanced Technologies and Health Systems for Underserved Populations (PATHS-UP) NSF Engineering Research Center. The UCLA Nanoelectronics Research Facility provided access to the fabrication equipment used for the study. Lin, Yu, Sabet, Di Carlo and Emaminejad have submitted a patent application through the UCLA Technology Development Group for the technology used in the new system.
