Greening Up the Production of Ammonia — the Key Ingredient for Fertilizer
UCLA chemical engineers co-lead research that could make industrial processes more sustainable
Katherine Steinberg/MIT
An illustration of high-resolution imaging of electrocatalysis with cryo-EM to reduce carbon emissions in ammonia production
UCLA Samueli Newsroom
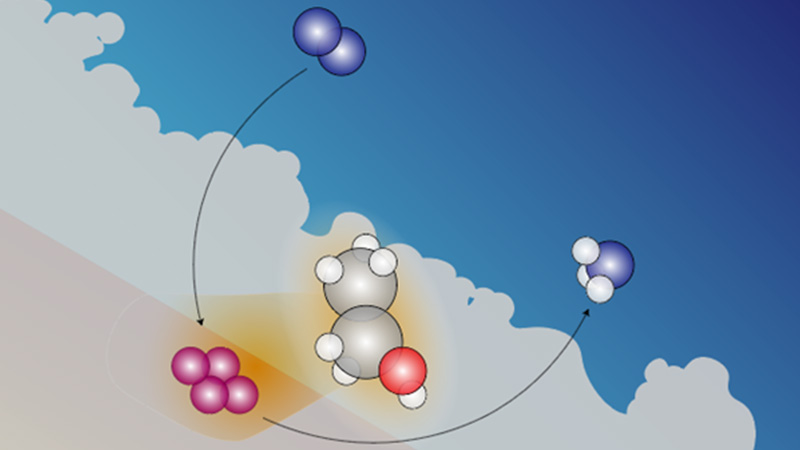
Ammonia may be best known in everyday life as a pungent household cleaning ingredient, but most of the inorganic compound produced each year is used in fertilizer, a vital resource for farming, and hence the production of ammonia also impacts the worldwide food supply.
Unfortunately, making this crucial compound of nitrogen and hydrogen also contributes to climate change. Today’s high-temperature, high-pressure industrial processes release carbon dioxide as a byproduct — almost twice as much by mass compared to the amount of ammonia produced. This accounts for an estimated 1 to 2% of global carbon dioxide emissions.
Now, research from UCLA, Caltech and MIT chemical engineers may help unlock a more sustainable approach to making ammonia at room temperature through electrocatalysis, a reaction that uses electricity alongside a chemical catalyst. Detailed in a study recently published in Nature Energy, the new technique leverages a high-resolution imaging technology that enabled the researchers to peer down to atomic level and understand the step by-step production of ammonia.
“Synthesizing ammonia and other commodity chemicals with electrocatalysis has the potential to help decarbonize chemical manufacturing,” said study co-corresponding author Yuzhang Li, an assistant professor of chemical and biomolecular engineering at the UCLA Samueli School of Engineering. “It also could offer a decentralized path for the wider production of ammonia. Rather than relying on a few industrial locations, this important chemical could be made at smaller facilities, as the special equipment in current use would no longer be needed.”
To get an up-close look at the ammonia-forming reaction, the research team employed cryogenic transmission electron microscopy, or cryo-EM. Considered to have revolutionized the field of structural biology, cryo-EM bounces electrons off of frozen samples to paint an atomic-level picture of details too small for light to capture. The technique was so groundbreaking that it garnered its developers the 2017 Nobel Prize in Chemistry.
Typically, cryo-EM has been used to reveal the molecular structure of proteins and other key molecules of life. This study represents the first time that cryo-EM was used to see materials in an electrocatalytic reaction. The technique enabled the researchers to overcome a previous limitation in understanding the reaction — the inability to see the surface of the catalyst molecule.
“Synthesizing ammonia and other commodity chemicals with electrocatalysis has the potential to help decarbonize chemical manufacturing,” said Yuzhang Li.
“I first utilized this powerful technique back in 2017 to uncover new findings on how batteries operate and fail at the atomic level,” Li said. “Now, my group has leveraged this technique to demonstrate its potential for research on electrocatalytic materials.”
Electrocatalysis can be used to transform readily available compounds, such as oxygen and carbon dioxide, into more complex molecules, such as methane and ammonia. The reaction that the research team watched play out at the molecular level, moment by moment, produces ammonia from the element lithium. The metal is put into a solution with nitrogen and a type of alcohol as primary ingredients.
Using cryo-EM, the researchers discovered a hitch in the reaction: A film of corrosion formed on the surface of the lithium, blocking nitrogen molecules from reaching it and therefore interfering with electrocatalysis. This is where the alcohol played its role, disrupting the corrosion and allowing ammonia to form.
Understanding how these molecules move around and interact is an essential step to enabling the widespread use of electrocatalysis to manufacture ammonia — and ultimately cutting into its carbon footprint.
The other co-corresponding author on the study is Karthish Manthiram, a Caltech professor. The co-first authors are doctoral students Katherine Steinberg of MIT and Xintong Yuan of UCLA. Other authors are graduate students Channing Klein and Nikifar Lazouski from Caltech and MIT respectively; and Matthew Mecklenburg, managing director of the Electron Imaging Center for Nanomachines at the California NanoSystems Institute at UCLA.
The research was supported by grants from the National Science Foundation.
