By Marlys Amundson
Professor Chih-Ming Ho and graduate student researchers Nan Li and Charlotte Kwong in the NASA-sponsored Institute for Cell Mimetic Space Exploration are developing a system here on Earth for monitoring astronauts’ health on manned space flights.
“As we travel farther into space, the need for smaller, lighter tools becomes even more critical,” said Ho. “Current blood testing systems are too bulky for space travel, so astronauts have to take samples while still in space and bring them back to Earth for analysis.”
The collaborative project, funded by the NASA National Space Biomedical Research Institute is led by Ho, professor Yu-Chong Tai at the California Institute of Technology, and Harvey Kasdan PhD ’71, chief scientist at IRIS International, Inc.
Current methods for analyzing blood chemistry require bulky equipment, lab technicians and several milliliters of blood. None of which are possible on board a space craft for routine health monitoring or diagnosis of disease.
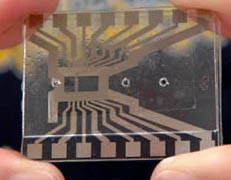 The size of a cell phone or PDA, the fully-automated testing system under development will require only a drop of blood and provide real-time clinical analysis. Such a lab-on-a-chip integrates all of the required laboratory functions onto a single integrated circuit that can be housed in a handheld unit.
The size of a cell phone or PDA, the fully-automated testing system under development will require only a drop of blood and provide real-time clinical analysis. Such a lab-on-a-chip integrates all of the required laboratory functions onto a single integrated circuit that can be housed in a handheld unit.
The UCLA researchers are developing tools to measure the distribution of white blood cells in humans-there are five different types-as a way to gauge the health of astronauts during space travel. Members of Ho’s lab are developing a micromachine system, which includes two main sub-systems, to manipulate the cells-one to remove ions from the sample so that the fluid can be manipulated with an electrical field and another to differentiate and count different groups of blood cells.
“The prototype microdeionizer we have now is capable of removing more than 90 percent of the ions in about 20 minutes,” said Kwong. “The two key elements in the device are a carbon aerogel electrode and a dialysis membrane.”
After a current is applied to the electrodes, an electric field is created that forces the ions into the buffer channels along the electric field. By developing an active method of ion extraction, Kwong has substantially reduced the amount of time needed to remove ions from a blood sample.
“Ion removal can be done solely by diffusion, which is passive, but it will take hours, if not days, to reach the desired ion concentration,” she noted. “And we chose carbon aerogel for our electrode material because it allows us to design a compact deionizer instead of having to build a huge tank.”
Carbon aerogel offers a significantly increased surface area in a compact system. For example, a 1.7 square millimeter of carbon aerogel paper has an equivalent surface area of 12 square meters.
Her colleague Li is using hydrodynamics to move the cells into a narrow channel so that only one cell at a time can move past an integrated micro impedance detector to distinguish among the five types of white blood cells in a sample.
In analyzing blood chemistry, cell counting and differentiation of the various types of white blood cells is a useful tool. To limit errors in analysis, however, the team needs to ensure that only one cell is scanned at a time.
“We used a hydrodynamic focusing technique in our device to focus the blood sample into a seven to 10 micrometer stream,” explained Li. “This is the dimension of white blood cells, and guarantees that only one can pass through the detecting zone at a time.”
Unlike conventional fluorescence-activated cell sorting (FACS) methods, which require cell labeling and a complex optical system, the UCLA system uses impedance spectroscopy, which applies a fast impedance scan within a wide frequency range to differentiate the cells. It is a non-invasive and label-free method that is compatible with micromachining technology.
Having developed several working prototypes, the UCLA team is developing a method to accurately differentiate between white blood cells with very similar electrical properties.
“We also need to find a way to treat the surface of the microfluidic device where the blood droplet is deposited to avoid contamination and inaccurate results,” added Ho.
To complete the lab-on-a-chip blood test system, Caltech is developing microfluidic devices to separate red cells from white cells. Kasdan is leading the IRIS team on system components and applying the company’s expertise to assemble the devices into a single working system.
Once developed, the system will have applications on Earth as well. Because of the small quantity of blood required and system portability, the unit may be used in neonatal units, as well as in remote locations. The collaborators also are interested in developing other lab-on-a-chip units for DNA analysis and other uses.
Main Image: a microdeinonizer. Inset Image: A lab-on-a-chip.
