A pair of researchers from the UCLA Henry Samueli School of Engineering and Applied Science has created the first surface texture that can repel all liquids, no matter what material the surface is made of.

Because its design relies only on the physical attributes of the texture, the texture could have industrial or biomedical applications. For example, the surface could slow corrosion and extend the life of parts in chemical and power plants, solar cells or cookware.
Water will bead up on a nonstick cooking pan because it is coated with a hydrophobic material that repels water thanks to its chemical composition. If the hydrophobic material also is rough at the microscopic scale, it can trap air at its surface, causing the water to bead up and roll around effortlessly. Scientists have named such surfaces “superhydrophobic” to distinguish their unusual zeal to repel water. As an example in nature, water droplets will bead and roll down on some leaves.
“At the microscopic scale, the leaves’ surfaces are ‘hairy’ and points of contact with water are reduced,” said Chang-Jin “CJ” Kim, a UCLA professor of mechanical and aerospace engineering, and the study’s principal investigator. “This reduction in points of contact means the water is held up by its own surface tension. Manmade superhydrophobic surfaces have been designed to take advantage of this phenomenon by forming microscale roughness or patterns on a hydrophobic material.”
While a nonstick cooking pan is hydrophobic, it is not “oleophobic,” meaning that it does not repel oil-based liquids. Cooking oil spreads out rather than beading up because it has a lower surface tension than water, making it more difficult to repel. Since the material is not oleophobic, roughening it won’t make its surface oleophobic, let alone “superoleophobic.”
However, in recent years scientists have created certain microscopic textures capable of making surface hydrophobic materials’ surfaces not only oleophobic but also superoleophobic.
But a true “omniphobic” surface — one that can repel any liquid, even those with the lowest surface tensions — has remained elusive.
Liquids with extremely low surface tension will “wet” not only the cooking pan but also even the best-performing superoleophobic surfaces today, collapsing into their microscopic texture. These liquids include fluorinated solvents, some of which are used for industrial applications like cooling electronic devices. Although the term “superomniphobic” began to be used by some, no surface was shown to repel the fluorinated solvents.
Working with Tingyi “Leo” Liu, a postdoctoral scholar in Kim’s lab and the paper’s lead author, Kim demonstrated for the first time true omniphobicity. The engineers formed a surface covered with thousands of microscale flathead nails, each about 20 micrometers in head diameter — each much smaller than the width of a typical human hair — resembling the appearance of existing superoleophobic textures.
The effect had never previously been observed, either on manmade or natural surfaces. It relies solely on the physical attributes of the texture, rather than any chemical properties of the material the surface is made of. Kim said it would actually be appropriate to call it a “mechanical” surface.
The research, which was part of Liu’s doctoral dissertation at UCLA, is published in the journal Science.
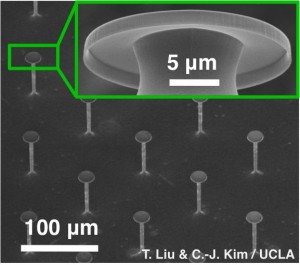
The key to the team’s innovative design is additional nanoscale details around the nail heads. Underneath the flat head, a nanoscale thin and short “curtain” surrounds the top and droops down vertically. This overhang creates a reverse meniscus when the liquid is on the surface and suspended between the nails. These special nails, spaced about 100 micrometers apart, are reminiscent of a serif letter “T” in cross section. On this engineered surface, even completely wetting liquids roll around like a ball and slide right off when the surface tilted.
“In a manned spaceship, you can see how a liquid will hold together as a sphere and that’s because it’s completely surrounded by air, that’s the same idea here,” Liu said. “On our textured surface, liquid sits on a cushion that is 95 percent air, and its own surface tension holds it up so it can roll over the surface without collapsing.”
The surface super-repelled all available liquids, including water, oils and many solvents, qualifying to be superomniphobic. It even super-repelled a fluorinated solvent called perfluorohexane, the liquid with the lowest known surface tension.
The team made the same microscale pattern on surfaces of glass, a metal and a polymer. In each case, the engineered surface super-repelled all liquids in a series of tests.
The researchers said it could be capable of lasting a long time in an outdoor environment, such as on buildings or vehicles, because its repelling properties would not degrade from ultraviolet light exposure and extreme temperatures. And it could improve biomedical devices because its repelling properties would not degrade because of fouling by biofluids.
Kim also has a UCLA faculty appointment in bioengineering and is a member of the California NanoSystems Institute. The researchers have filed a patent on the work.
Main image: Water, methanol and FC-72, a fluorinated solvent, rolling around on the UCLA researchers’ superomniphobic surface.
