UCLA Chemists and Chemical Engineers Demonstrate Promising Method for Faster Drug Discovery
UCLA Nelson and Tang research groups
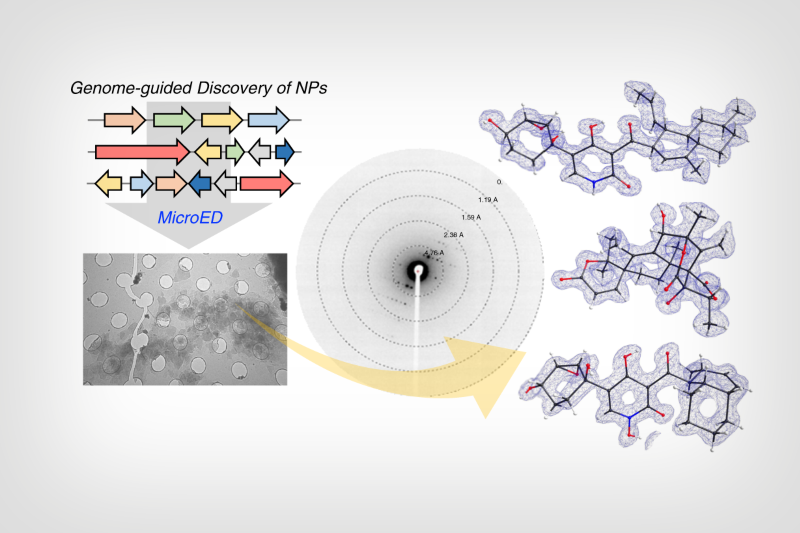
Diagram showing how synthetic biology techniques coupled with an imaging technique called microcrystal electron diffraction can lead to the discovery of natural products
UCLA chemists and chemical engineers have combined two scientific techniques – one that sifts through mountains of complex genomic data and another that images molecular structures in unprecedented 3D detail – to demonstrate a powerful new pathway towards an expedited discovery of new drugs.
The research was recently published in Nature Chemical Biology and highlighted by the UCLA Department of Chemistry & Biochemistry.
UCLA researchers focused their study on natural products – molecules produced by living organisms such as bacteria, fungi and plants. These structurally complex molecules can be front-line pharmaceuticals for preventing and treating human diseases. For example, the antibiotic erythromycin and the chemotherapy drug paclitaxel (known by the brand name Taxol) are both natural products.
However, due to the scarcity of these products in nature, it takes tremendous time and effort to isolate and characterize their molecular structures, which can impede their discovery.
To address this bottleneck, the researchers first used synthetic biology techniques to find and isolate genes available in a massive online library that produce enzymes from fungi. These enzymes are then screened to see what new compounds they help produce. The technique also works for compounds that are “orphaned” due to unavailability of the original reported organism.
Then, the team used an emerging technique, called microcrystal electron diffraction, or MicroED, to image and rapidly configure the complete, three-dimensional structures of the newly isolated molecules.
Knowing the genes that code for a natural product along with its resulting structure can shed light on its capabilities, including the potential of being a promising drug. The next step is to speed up the process and broaden its scope to expedite the discovery of more promising natural products.
When combined together, the synthetic biology and MicroED techniques will enable rapid discovery of new natural products, which will then accelerate drug discovery, according to the UCLA team.
Team members on the research include co-lead authors Lee Joon Kim, a doctoral student in chemistry and biochemistry, and research scientist Masao Ohashi of chemical and biomolecular engineering. The co-corresponding authors are Yi Tang, UCLA’s Ralph M. Parsons Foundation Professor in Chemical Engineering; and Hosea Nelson, an associate professor of chemistry and biochemistry. Other UCLA co-authors of the study are former postdoctoral scholars Zhuan Zhang and Matthew Asay; visiting scientist Dan Tan; senior staff scientist Duilioi Cascio; and José Rodriguez, an associate professor of chemistry and biochemistry.
The authors used the X-ray Crystallography Core Facility at UCLA, a joint institute supported by the U.S. Department of Energy, to conduct some of the research. The team was supported by grants from the Department of Energy, the National Science Foundation and the National Institutes of Health, with additional support from the David and Lucille Packard Foundation, the Pew Charitable Trusts, the Beckman Foundation and a research grant from Bristol Myers Squibb.
Penny Jennings, of UCLA Department of Chemistry & Biochemistry, contributed to this story.
