Bioengineers Find Ways to Enhance Potential Drug for Chronic Inflammatory Diseases
Aaron Meyer Laboratory/UCLA
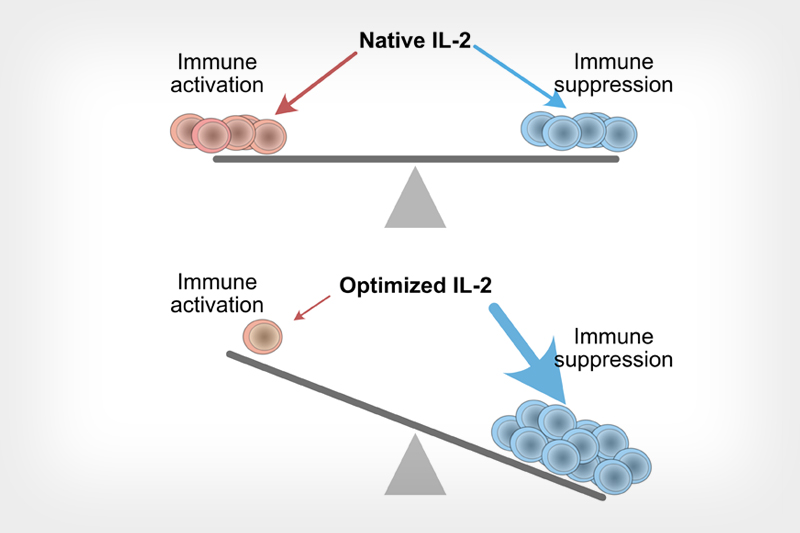
Diagram shows an artificially optimized cytokine protein IL-2 can rebalance its effects toward suppressing the immune system’s activity.
UCLA bioengineers have created a computational model to fine-tune a potential drug for the treatment of chronic inflammatory diseases. A study detailing the finding was recently published in Cell Reports.
The researchers focused on a naturally produced cytokine protein, IL-2. IL-2 sends signals to the immune system and helps regulates its level of activity. Depending on the dose and modifications of IL-2, it can either improve the immune system’s activity or dampen it. IL-2 has beneficial effects as a potential drug but can also be toxic in some variants. Thus far, the only approved IL-2 therapy by the U.S. Food and Drug Administration is for melanoma treatment to help drive a stronger anti-cancer immune response. This type of treatment occurs under supervision at a hospital using high doses for about a week each round.
A research team led by Aaron Meyer, an assistant professor of bioengineering at the UCLA Samueli School of Engineering, built a computational model demonstrating how IL-2 and engineered variations of the molecule interact with cells in the immune system, and the potential outcomes of those interactions.
Subtle modifications to IL-2’s molecular structure could dramatically lessen its toxicity.
Many anti-inflammatory drugs are prescribed for life so the toxicity of IL-2 could be a major hurdle for more widespread adoption of similar drugs based on the protein. However, subtle modifications to IL-2’s molecular structure could dramatically lessen its toxicity. Drugs based on a modified form with reduced toxic side effects would allow for its long-term use, potentially outside of a hospital setting.
“We’ve done a much more tightly integrated computational and engineering analysis of IL-2 than previous research attempts,” Meyer said. “We have also taken a more comprehensive approach in our experimental characterization. This allowed us to identify engineering subtleties that have important consequences on the potential of therapies that use this cytokine.”
For example, Meyer said the team observed that a seemingly small change in how a drug is constructed significantly changes its effect on cells. With the right engineering, the researchers are hopeful that people might be able to self-administer the drug at home rather than in a hospital.
The co-lead authors of the study are former UCLA bioengineering undergraduates Ali Farhat and Adam Weiner. Farhat is now in an M.D.-Ph.D. program at the University of Illinois-Chicago and Weiner is a graduate student in a multi-institutional computational biology program run by Cornell University and its medical center, Rockefeller University, and Memorial Sloan Kettering Cancer Center. Other UCLA authors are undergraduate student Zoe Kim and graduate student Brian Orcutt-Jahns. Additional authors are Cori Posner and Scott Carlson of Visterra, a biotechnology company in Massachusetts.
The research was supported by the National Institutes of Health and a research agreement with Visterra.
